Positive Pressure or Negative Pressure? A Standard Guide to Cleanroom Design
09:50 - 04/04/2025 1967
Positive Pressure or Negative Pressure? A Guide to Proper Cleanroom Design According to ISO 14644 and GMP, Ensuring Contamination Control and Production Efficiency.
Factors affecting the recovery of microorganisms from surfaces using contact plates in cleanrooms.
Types of cleanroom walls: Comparison and how to choose the right one
Advanced therapy medicinal product cleanroom facilities (ATMPs): A roadmap to success.
Understanding the difference between cleanroom certification and cleanroom validation.
Introduction
In cleanroom design, the choice of positive pressure or negative pressure is a technical factor that determines safety, efficiency, and contamination control. This is not only a mechanical issue but also relates to industry standards such as ISO 14644, WHO GMP, FDA 21 CFR Part 11 and the specific requirements of various fields such as pharmaceuticals, medical devices, or microbiology.
This article is a guide to proper cleanroom design, helping you understand the types of pressures, how to choose, and how to arrange the system according to the intended use.
Distinguishing Positive and Negative Pressure in Cleanrooms
What is Positive Pressure?
Positive pressure occurs when the air pressure inside the cleanroom is higher than the outside. The airflow always moves from the inside to the outside, preventing contaminants from entering.
Applications:
- Pharmaceutical production cleanrooms
- Injection drug preparation areas
- Medical device production cleanrooms
What is Negative Pressure?
Negative pressure is when the air inside the room has lower pressure than the outside. Air is drawn in rather than pushed out.
Applications:
- Biological isolation rooms
- Hazardous chemical processing areas
- Storage for infectious materials
When to Choose a Cleanroom with Positive or Negative Pressure?
Purpose | Pressure Type | Reason |
Protecting products from contamination | Positive pressure | Prevents dirty air from entering |
Protecting the environment from hazardous substances | Negative pressure | Keeps substances contained within |
Note: Some areas use a combination of positive and negative pressure, creating controlled pressure differentials between rooms.
Cleanroom Pressure Differential Systems and Their Role in Design
Standard cleanroom design must ensure:
- A minimum pressure differential of 10–15 Pa between rooms of different cleanliness levels (according to ISO 14644)
- Use of a synchronized HVAC system with pressure sensors and a cleanroom pressure controller
- Installation of a pressure differential gauge at the entry door for monitoring
- Integration of a cleanroom pressure display system on the control panel or BMS
Pressure Differential Layout:
- Central cleanroom (highest pressure)
- Surrounding rooms with gradually decreasing pressure
- Intermediate corridor/diffusion area maintaining a differential of 5–10 Pa
Pressure Standards in GMP Cleanrooms
According to the WHO GMP cleanroom standard and EU GMP, the required pressure differentials are:
- Between Grade A and B rooms: ≥15 Pa
- Between Grade B and C rooms: ≥10 Pa
- Between production rooms and corridors: ≥10 Pa
Class 100 cleanrooms typically maintain an air pressure at least 12.5 Pa higher than the surrounding environment to ensure airflow outward.
How to Effectively Control Cleanroom Pressure
- Required Equipment:
- Pressure differential sensors: measure pressure in real time
- Pressure controllers: manage the airflow in and out
- Mechanical or digital pressure gauges
- A precise HVAC system capable of adjusting airflow by zone
- Sensor Installation Locations:
- At the HVAC inlet
- At the main entrance of the cleanroom
- At partition walls
- Regular Inspections:
- Measure pressure daily before and after production shifts
- Record any abnormal fluctuations for timely adjustments
Cleanroom Classification by Pressure – Practical Applications
Field | Pressure Type Used | ISO Grade |
Sterile pharmaceuticals | Positive pressure | ISO 5–7 |
Medical devices | Positive pressure | ISO 7–8 |
Microbiology/virology | Negative pressure | ISO 6–8 |
Testing laboratories | Combined | ISO 6–8 |
What Should Be Noted in the Design of Pharmaceutical Cleanrooms?
- Arrange airflow from the cleanest to less clean areas
- Avoid reverse airflow from low-pressure to high-pressure areas
- Integrate an alert system if the pressure differential exceeds the allowed threshold
Frequently Asked Questions about Pressure Differentials and Cleanroom Pressure
What is positive pressure in cleanroom design?
It is a higher pressure than the outside environment, pushing clean air out to prevent contamination from entering.
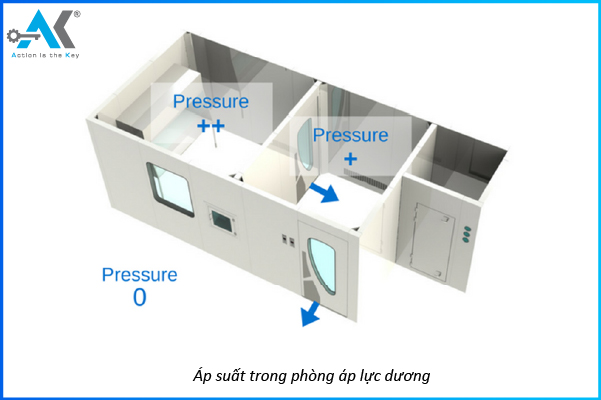
When should a cleanroom with negative pressure be used?
When it is necessary to contain air or contaminants within the room, preventing them from spreading to the outside environment.
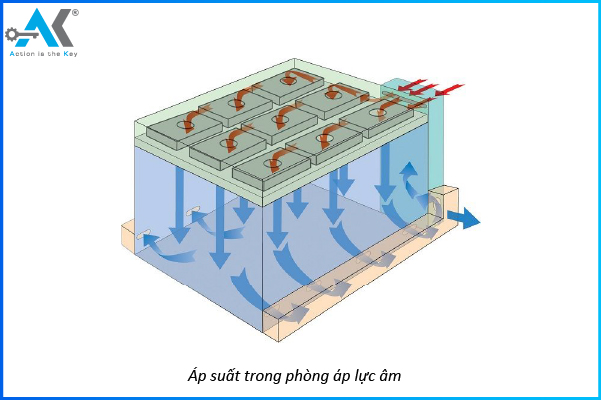
How to adjust the pressure differential between cleanrooms?
Use a flow controller, adjust fan speed, or modify the HVAC output pressure in each room.
What pressure differential is considered GMP compliant?
Between 10–15 Pa depending on the location, following the guidelines of ISO 14644 and GMP.
Do cleanrooms require continuous pressure monitoring?
They should be monitored continuously or at least per production shift. A BMS or digital device helps with real-time monitoring.
Conclusion
Positive pressure or negative pressure? A guide to proper cleanroom design is a core factor in building a standard cleanroom system. Depending on the intended use and industry, you need to determine the appropriate pressure type and maintain a stable differential to ensure quality and production safety.
Proper design from the start will help businesses save costs, achieve easier certification, and avoid future operational risks.
For consultation, design, and construction of cleanrooms, please contact Anh Khang Cleanroom at:
 | ANH KHANG CLEANROOM JOINT STOCK COMPANY Hotline: 1900 636 814 - 0902 051 222 Email: info@akme.com.vn Website: akme.com.vn Address: Lot B7 - Xuan Phuong Garden - Phuong Canh - Nam Tu Liem - Hanoi. |
12:05 - 28/11/2019 47755
Cleanroom Design and Construction
14:05 - 11/03/2025 21692
GMP and ISO Standard Cleanroom Construction
14:18 - 11/03/2025 12232
ISO Standard Medical Cleanroom Construction
14:13 - 28/02/2025 23360
Electronics Cleanroom Construction
16:15 - 18/03/2021 5602
Warranty Service
16:26 - 28/11/2019 18464
Supply and installation of cleanroom equipment
14:50 - 26/11/2019 6140
Technology Production Line Consulting
16:35 - 19/03/2025 19149














