Secrets of Pharmaceutical Cleanroom Construction: Ensuring Absolute Sterility
10:23 - 03/03/2025 3201
Pharmaceutical cleanroom construction that meets GMP/WHO standards helps control sterility and minimize contamination. Discover the secrets to ensuring quality from the very start.
State of the art Cleanroom Equipment in the Pharmaceutical Industry
Pharmaceutical Cleanroom Construction
The Importance of Cleanrooms in the Pharmaceutical Industry
In the pharmaceutical industry, cleanrooms are not only a technical requirement but also a decisive factor for product quality and production safety. Complying with CGMP and adhering to the WHO GMP standards is indispensable for ensuring that the cleanroom meets the criteria.
In addition, in the context of the constantly evolving pharmaceutical industry, the application of Cleanroom Applications in Food is also being explored to ensure hygiene safety in related sectors.
If Pharmaceutical Cleanroom Construction is not carried out according to the proper procedures, businesses may face serious issues:
- Products rejected due to microbial contamination, failing to meet market standards
- Risk of production line shutdown due to violations of GMP regulations
- High maintenance and repair costs due to non-compliant design and construction
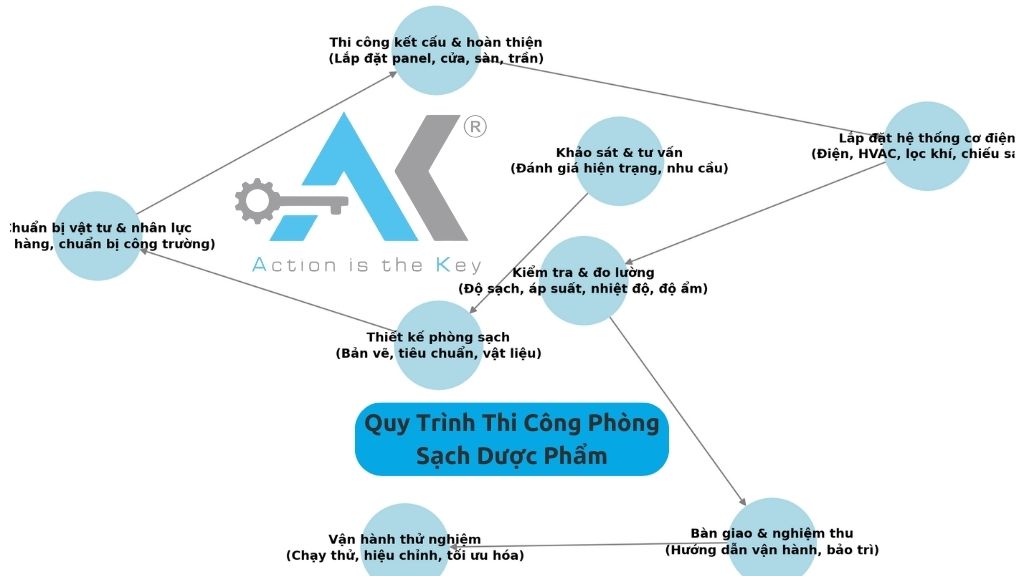
So how can pharmaceutical cleanroom construction meet GMP/WHO standards from the very beginning? Let’s explore the key implementation steps and methods to ensure absolute sterility.
_1.jpg)
Steps for Constructing Pharmaceutical Cleanrooms to GMP Standards
Planning and Designing the Cleanroom
Before construction, it is necessary to clearly determine the important factors:
- Cleanliness Level: Standard ISO 14644 -1, depending on the level of microbial control in each area
- Pressure Control System: Cleanrooms with positive pressure to prevent contamination, and negative pressure to handle highly toxic pharmaceuticals
- HVAC & Air Filtration System: Controls temperature, humidity, and air circulation speed to ensure sterility
Businesses should choose a reputable partner for Cleanroom Design to establish a solid foundation from the design stage, helping to control cross-contamination, optimize production efficiency, and reduce operating costs.
Constructing the Cleanroom System
To effectively carry out Cleanroom Construction, it is necessary to follow the proper procedures from installation to pressure and airflow control.
1️⃣ Install wall, ceiling, and floor panels:
- Use antibacterial, dust-resistant panels, ensuring a sealed structure
- Establish an anti-static flooring system if necessary
2️⃣ Construct the HVAC & Air Filtration System
- Install HEPA/ULPA filters, ensuring the removal of 99.99% of bacteria and fine dust
- Control pressure differentials between areas to prevent air leakage
3️⃣ Set up the Pressure and Airflow Control System
- Monitor cleanroom pressure, ensuring no intrusion of outside air
- Install differential pressure gauges, to track pressure conditions in real time
4️⃣ Install Supporting Cleanroom Equipment
- Pass Box, Air Shower, UV light system, to help minimize dust and bacteria when personnel enter and exit the cleanroom
- Automatic doors, interlock systems, ensuring absolute sterility of the environment
Furthermore, the use of Pharmaceutical Cleanroom Equipment will contribute to enhancing control efficiency and ensuring system reliability.
See more: Air Pressure Control Systems in Cleanrooms
Controlling Bacteria & Ensuring Sterility in the Cleanroom
After construction is complete, the cleanroom must undergo rigorous testing procedures to ensure it meets the standards:
- Measure air cleanliness levels according to ISO 14644 standards
- Check airflow speed, pressure, temperature, and humidity, to ensure a stable environment
- Sterilize using specialized chemicals, eliminating 99.99% of bacteria and mold
For example: A pharmaceutical plant in Ho Chi Minh City experienced a product contamination incident due to a low-quality air filtration system. After being upgraded by ANH KHANG CLEANROOM, the plant reduced its contamination risk by 90%, ensuring a safe production process.

ANH KHANG CLEANROOM – Your Trusted Cleanroom Construction Partner
Pharmaceutical cleanroom construction is a complex process that requires absolute precision to ensure product quality, compliance with GMP/WHO, and production safety.
To meet standards from the very beginning, ANH KHANG CLEANROOM provides comprehensive services including consultation, design, and construction, helping businesses optimize costs, maintain stable cleanliness, and enhance operational efficiency.
Furthermore, for other sectors such as healthcare, businesses may refer to the Medical Cleanroom Construction service to ensure the production environment always meets safety standards.
In particular, for a comprehensive and effective solution, please contact ANH KHANG CLEANROOM for a Consultation on Pharmaceutical Cleanroom Construction.
 | ANH KHANG CLEANROOM ELECTRICAL JOINT STOCK COMPANY Hotline: 1900 636 814 Email: info@akme.com.vn Website: akme.com.vn Address: Lot B7 Xuân Phương Garden, Trinh Van Bo Street, Phuong Canh Ward, Nam Tu Liem District, Hanoi. |
12:05 - 28/11/2019 47755
Cleanroom Design and Construction
14:05 - 11/03/2025 21691
GMP and ISO Standard Cleanroom Construction
14:18 - 11/03/2025 12232
ISO Standard Medical Cleanroom Construction
14:13 - 28/02/2025 23360
Electronics Cleanroom Construction
16:15 - 18/03/2021 5602
Warranty Service
16:26 - 28/11/2019 18463
Supply and installation of cleanroom equipment
14:50 - 26/11/2019 6140
Technology Production Line Consulting
16:35 - 19/03/2025 19149














