GMP or ISO 14644: Which Standard is Optimal for Medical Cleanrooms?
12:50 - 03/03/2025 5112
A Comparison of Differences, Implementation Methods, and the Appropriate Choice for Hospitals & Pharmaceutical Plants.
Medical Cleanroom: The Golden Secret to Maintaining Sterility and Optimizing Operational Efficiency
Comprehensive Hospital Air Quality Testing Solution
Anti-Static Clean Room: Protecting Medical Equipment (Testing Machines, X-Ray)
Modern Hospital Cleanroom Design
The Importance of Medical Cleanroom Standards
In the field of pharmaceuticals & healthcare , ensuring a sterile environment is a prerequisite for protecting patient health and maintaining product quality. Controlling the air cleanliness, microorganisms, pressure differences, and other physical factors plays a crucial role in pharmaceutical factories, hospitals, and laboratories. The application of Air Testing Technology contributes to enhanced control efficiency. At the same time, the process of ensuring a sterile process environment is also a key factor.
The two most important standards in medical cleanroom control are:
- GMP Medical Cleanroom Standard – focusing on sterile production processes, preventing cross-contamination in the pharmaceutical & medical device industries.
- ISO 14644 – ensures particle control and air flow management, helping cleanrooms operate effectively.
Choosing the appropriate standard helps enterprises comply with regulations, optimize operational efficiency, and avoid unnecessary risks .

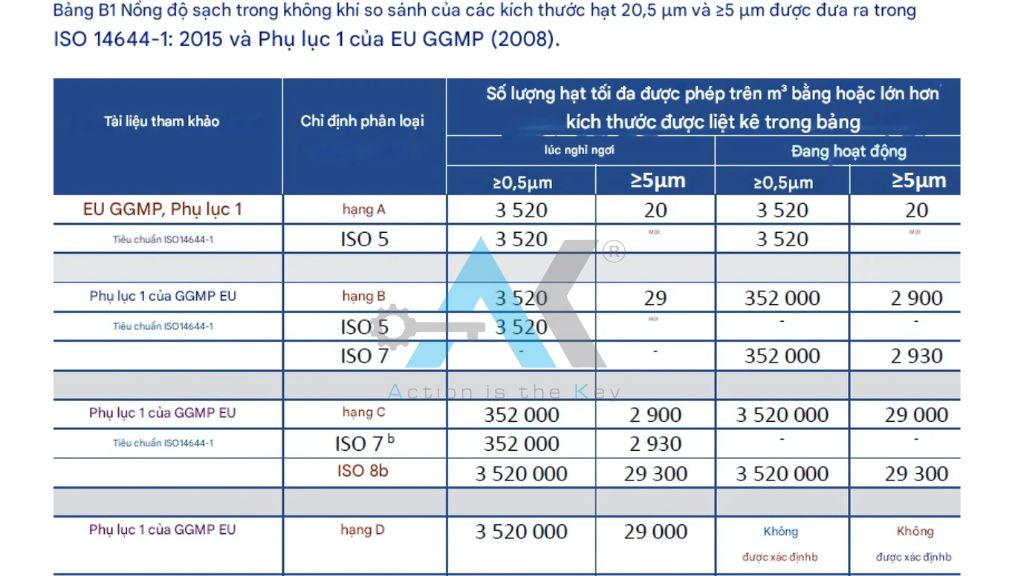
Comparing GMP & ISO 14644 in Medical Cleanrooms
The Differences Between GMP & ISO 14644
Criteria | GMP | ISO 14644 |
Main Objective | Ensure sterility, microbial control, and the production process for pharmaceuticals & medical devices | Focuses on air cleanliness, particle control, and cleanroom classification . (ISO 14644) |
Scope of Application | Pharmaceutical factories, hospitals, microbiology labs | Industrial cleanrooms, medical devices, electronics manufacturing |
Control Requirements | Microorganisms, humidity, pressure differences, cross-contamination control | Airborne particles, airflow speed, cleanliness levels ISO 1-9 |
Applicable Standards | WHO-GMP, EU GMP, US-FDA | ISO 14644-1, ISO 14644-2 |
Inspection & Acceptance | Evaluated through the GMP Audit process , confirming sterility | Particle measurement, cleanroom operational assessment |
Practical Applications of GMP & ISO 14644
When to Apply GMP?
- Pharmaceutical factories manufacturing drugs, vaccines, medical devices
- Hospitals with operating rooms, emergency intensive care units, drug preparation cleanrooms
- Laboratories conducting microbiological research, pharmaceutical testing
GMP helps to strictly control microorganisms and ensure that pharmaceuticals & medical devices remain contamination-free , reducing the risk of cross-contamination.
When to Apply ISO 14644?
- Cleanrooms for manufacturing medical devices, conducting microbiological tests
- Hospitals with diagnostic imaging and X-ray testing areas
- Electronics factories, high-tech component manufacturing
ISO 14644 helps to control the number of airborne particles , preventing particle contamination that could affect medical devices and products.

To ensure maximum safety, Electrostatic Discharge Cleanrooms are also an important consideration.
Choosing the Appropriate GMP ISO Standard
A hospital in Hanoi once encountered a situation with high infection rates in its operating rooms , leading to a 30% increase in postoperative infections . The main cause was that the cleanroom did not meet the GMP standards of Hospital Cleanrooms , with inadequate microbial control.
After upgrading the cleanroom system to meet WHO GMP standards, the hospital achieved:
✅ A 50% reduction in postoperative infection risks
✅ International medical standard compliance, enhancing hospital reputation
✅ Minimized cross-contamination, improved treatment quality
This demonstrates that applying the right standard according to the cleanroom's function helps optimize operations, ensure patient safety, and enhance medical effectiveness.
Enterprises may refer to our Medical Cleanroom Consulting Services.
In addition, to ensure construction quality, enterprises may also consider Cleanroom Construction.
Optimizing Medical Cleanroom Standards – The Right Investment
Both GMP and ISO 14644 are crucial in the design and operation of medical cleanrooms; however:
- GMP is a mandatory standard for pharmaceutical factories, hospitals, and laboratories.
- ISO 14644 ensures air cleanliness and is applied in medical testing and device manufacturing.
Choosing the right standard will help enterprises comply with regulations, optimize operations, and meet international standards.
ANH KHANG CLEANROOM specializes in Cleanroom Design , consulting, Medical Cleanroom Construction , helping enterprises ensure quality and sustainable operations.
 | CÔNG TY CỔ PHẦN CƠ ĐIỆN PHÒNG SẠCH ANH KHANG Hotline: 1900 636 814 - 0902 051 222 Email: info@akme.com.vn Website: akme.com.vn Add: Lô B7 - Xuân Phương Garden - Phương Canh - Nam Từ Liêm - Hà Nội. |
12:05 - 28/11/2019 47858
Cleanroom Design and Construction
14:05 - 11/03/2025 22343
GMP and ISO Standard Cleanroom Construction
14:18 - 11/03/2025 12302
ISO Standard Medical Cleanroom Construction
14:13 - 28/02/2025 23455
Electronics Cleanroom Construction
16:15 - 18/03/2021 5971
Warranty Service
16:26 - 28/11/2019 18804
Supply and installation of cleanroom equipment
14:50 - 26/11/2019 6207
Technology Production Line Consulting
16:35 - 19/03/2025 19469














