Why are strict cleanroom standards necessary for pharmaceuticals and healthcare?
09:50 - 03/03/2025 3206
Medical and pharmaceutical cleanrooms ensure sterility control and compliance with GMP and FDA standards. Discover the stringent regulations and professional cleanroom construction solutions now!
Cleanroom Air Control System
Clean Room Installation: Implementation Steps from A to Z
What does the clean room system consist of?
Clean Room Quality Monitoring and Inspection Process
The Importance of Sterility in the Pharmaceutical and Medical Industry
In the pharmaceutical and healthcare industry, clean rooms are not merely a technical standard but also a key factor determining the safety, efficacy, and quality of products. The strict regulations of GMP-WHO, FDA, ISO 14644, EU-GMP require the production environment to be absolutely sterile, minimizing the risks of contamination, cross-contamination and ensuring the safety of patients and customers.
At the manufacturing plants for drugs, vaccines, medical devices, and hospitals, even a small error in controlling air quality, pressure, and cleanliness can result in serious consequences, directly affecting the health of users and the reputation of the company.

For the pharmaceutical field, Pharmaceutical Clean Room Construction is always the top priority.
Consequences of Not Meeting Clean Room Standards in Healthcare and Pharmaceuticals
Failure to comply with strict clean room standards can lead to significant risks for companies and hospitals. Some serious consequences include:
1. Risk of Contamination & Cross-Contamination
- Pharmaceutical products contaminated with bacteria, mold, and airborne impurities.
- Cross-contamination between production lines or among patients in hospitals.
- Impacting the quality of medicines and reducing treatment efficacy.
Real-life example:
In 2021, an antibiotic manufacturing plant in Europe had a batch worth millions of USD recalled due to the detection of bacteria in the production environment, affecting millions of patients.
2. Violation of International Standards, Leading to Operational Suspension
- Failure to obtain certifications such as GMP-WHO, FDA, ISO 14644, causing companies to lose opportunities for export and market expansion.
- Leading to production suspension, revenue loss, and damage to brand reputation.
Real-life example:
A large hospital in Southeast Asia had its surgical unit closed due to a non-compliant sterile control system, causing treatment disruptions for thousands of patients.
3. Significant Financial Losses Due to Error Remediation
- The cost of rectifying clean room errors is 2-3 times higher than the initial investment cost.
- The entire system for air filtration, HVAC, and room pressure must be replaced if not compliant.
Real-life example:
A vaccine manufacturing company in Vietnam had to spend an additional 5 billion VND to upgrade the clean room after being assessed as not meeting GMP standards.

Benefits of Investing in a Compliant Clean Room System in the Healthcare and Pharmaceutical Industries
1. Ensuring the Quality of Pharmaceutical Products, Vaccines, Medical Devices
- Strict control of air cleanliness, pressure differentials, temperature, and humidity.
- Minimizes contamination, protecting the quality of medicines and vaccines.
2. Compliance with International Standards, Facilitating Export
- Obtaining certifications such as GMP-WHO, FDA, EU-GMP elevates the company's standing in the international market.
- Helps companies expand their market and enhance production capacity.
3. Minimizing Health Risks & Avoiding Litigation
- Prevents cross-contamination, ensuring absolute safety for patients.
- Reduces the risk of litigation due to non-compliant pharmaceutical products or medical devices.
4. Optimizing Long-Term Operating & Maintenance Costs
- A clean room system designed to proper standards from the outset helps companies save up to 30% on future repair and upgrade costs.
- Extends the lifespan of air filtration systems, HVAC equipment, and air pressure systems.
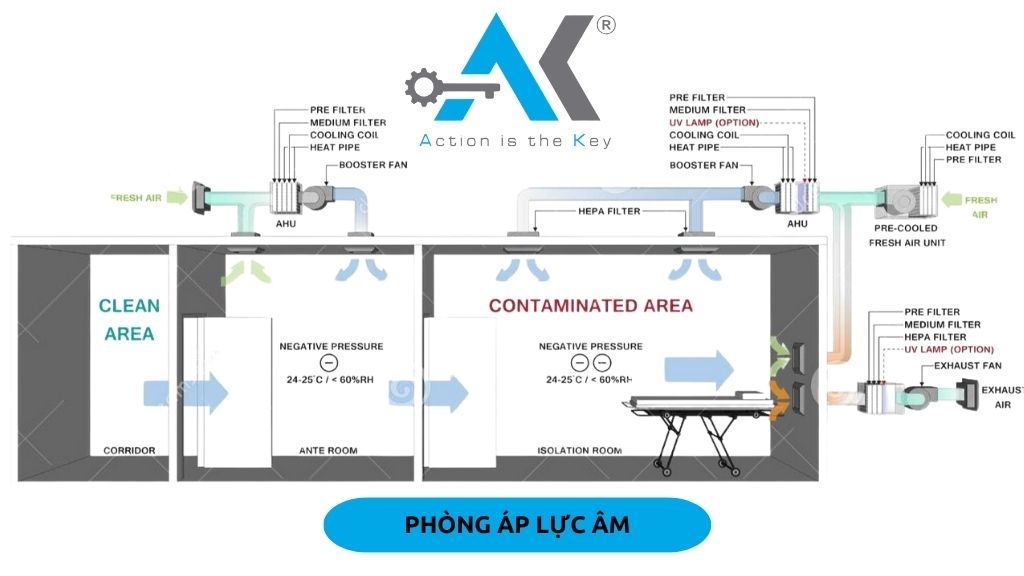
Key Factors in the Design of Clean Rooms for Healthcare and Pharmaceuticals
To ensure a healthcare-compliant clean room, companies need to pay attention to the following factors. Additionally, Healthcare Clean Room Construction is a key element in ensuring a compliant production environment.
Firstly, companies need to invest in professional Clean Room Construction solutions to ensure effective control of the production environment.
At the same time, a professional Clean Room Design system will help companies optimize operating processes, maintenance, and meet international standards.
- Positive Pressure: Used in production areas for injectable drugs, vaccines, antibiotics to ensure that clean air is not infiltrated by dust or bacteria.
- Negative Pressure: Used in areas handling hazardous chemicals, hospital isolation zones to prevent contaminated air from escaping.
- Insulated, Anti-Fouling Panels help maintain cleanliness.
- Automatic Door Systems, Air Shower, Pass Box help control the flow of movement.
- In a high-tech environment, the application of Electronic Clean Room Construction is evidence of the commitment to ensuring quality.
Furthermore, to complete the solution, companies need to invest in comprehensive Clean Room Design and Construction that integrates modern technologies with traditional production processes.
Companies in the field of Food Clean Room Construction can also learn and apply valuable insights from this modern clean room system.
Contact Now for Consultation on Healthcare and Pharmaceutical Clean Room Systems
Investing in compliant clean rooms not only helps companies adhere to international standards but also ensures product quality and public health. ANH KHANG CLEANROOM proudly stands as the leading provider of clean room consulting, design, and construction in the field of pharmaceuticals & healthcare, committed to:
✅ Design & install clean rooms compliant with GMP, FDA, ISO 14644
✅ Optimize air filtration systems, pressure, and airflow
✅ Ensure acceptance testing meets international standards
✅ Maintenance & upgrade services for clean room systems
Dear companies interested in healthcare & pharmaceutical clean room solutions, please contact ANH KHANG CLEANROOM now for detailed consultation!
 | ANH KHANG CLEANROOM ELECTRICAL CLEAN ROOM JOINT STOCK COMPANY Hotline: 1900 636 814 - 0902 051 222 Email: info@akme.com.vn Website: akme.com.vn Address: Lot B7 - Xuan Phuong Garden - Phuong Canh - Nam Tu Liem - Hanoi. |
12:05 - 28/11/2019 47755
Cleanroom Design and Construction
14:05 - 11/03/2025 21692
GMP and ISO Standard Cleanroom Construction
14:18 - 11/03/2025 12232
ISO Standard Medical Cleanroom Construction
14:13 - 28/02/2025 23360
Electronics Cleanroom Construction
16:15 - 18/03/2021 5602
Warranty Service
16:26 - 28/11/2019 18464
Supply and installation of cleanroom equipment
14:50 - 26/11/2019 6140
Technology Production Line Consulting
16:35 - 19/03/2025 19149














